Medical
Experience
Pentad Design has many years of experience designing a wide range of medical devices. We are fully aware of the requirements to design Class I, II, and III Medical Devices from product conception to production. This includes the initial design concept, planning, overall development, testing, optimization, and documentation. Risk management activities plus ongoing verification and validation testing are in accordance to the appropriate release markets and your quality system.
Our engineers have developed successful medical devices capturing highly profitable market leading positions for our client firms. Pentad Design has completed a number of projects from concept to creation and execution of validation procedures and records.
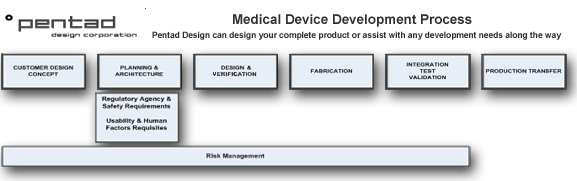
See diagram below for a brief synopsis of our medical design process.
Regulatory
Whether 510K or PMA requirements, US, European or Worldwide standards, our experience in regulatory and agency compliance is extensive and we maintain ongoing memberships and stay abreast of current updates with the regulatory agencies and medical instrumentation associations.
Reliability
We understand that reliability is a key concern in medical environments. Along with testing our code via standard verification and validation procedures, we use compilers and development environments which have been validated from independent test houses and commercial test suites. It is also standard procedure to use static analysis tools as another safeguard against poorly documented features of programming languages which might result in incorrect implementation by the compiler suite.
Products
Here is a partial list of the extensive number of products that Pentad Design has produced:
• Ventilators (ICU, Sub-Acute and Home Care)
• Respiratory Monitoring Devices
• In–Vitro diagnostic equipment
• Dental Diagnostic Devices
• Drug Delivery Products
• Smoke Evacuation Equipment
• Disposable and Reusable Electronics
• Ebeam sterilizable electronics
• Power Reduction – Battery Operated Medical Devices
Whether you require medical device product development from initial concept to production or require a Pentad team member to expedite your design time and technology requirements we can help you meet your goals.
Please contact us at the following number: (714)-259-0125 or use this link to communicate with us online. info@pentaddesign.com

